Integrating environmental effects in the benefit-risk assessment of therapeutic products: a proposal and example for sustainable health and healthcare

1 Introduction
Current global trends in healthcare indicate that environmental issues no longer can or should be ignored. Innovation and the inclusion of environmental aspects in the development, evaluation, and delivery of healthcare treatments are urgently needed. Specifically, environmental aspects need to be included in benefit-to-risk assessments and this expanded criterion should be adopted by all stakeholders in the healthcare value chain. With regards to healthcare delivery, healthcare providers can have a particularly unique role in improving its sustainability as decision-makers who can (should) make an informed choice about treatment choices in consultation with their patients. Each decision can be a step toward increasing market demand for treatments that are more environmentally friendly and decreasing the entry of pollutants into water systems.
The paradox between the aim of healthcare to improve health and how the environmental impact of providing healthcare puts human health at risk must be addressed (Lenzen et al., 2020; Halonen et al., 2021; Mortimer and Pencheon, 2022). While advances in healthcare over the past decades have improved human health, healthcare has a considerable ecological footprint and contributes to anthropogenic changes such as loss of biodiversity and destabilization of planet’s ecosystems. Consequences of biodiversity loss and destabilized ecosystems that threaten human health include extreme weather events, air pollution, food and water insecurity, and infectious diseases (Romanelli et al., 2015).
Another specific example of how the unintended environmental effects of healthcare affect human health involves our microbiota and antibiotics (Prescott et al., 2022; World Health Organization, 2022). There is a growing evidence that excreted antibiotics contaminate water, soil, and vegetables and alter their microbial ecosystem, favoring the development and growth of resistant bacteria (Carvalho and Santos, 2016; Li et al., 2022; Grenni et al., 2018; Robles-Jimenez et al., 2021; Maghsodian et al., 2022; Symochko et al., 2023; Cycoń et al., 2019). Any antibiotic-induced perturbation of composition and diversity of the microbiota involves humans, animals, plants, and the environment as they continuously exchange microbiota, and often leads to an increase in phyla with a high content of antimicrobial resistance genes. In the case of humans, these resistant microbial strains and antibiotic residues enter the body via consumed crops and water; the resistant strains in particular could subsequently affect gut microbiota (Miller et al., 2016; Hirt, 2020; Flandroy et al., 2018; De Filippo et al., 2017). As the gut microbiome can be regarded physiologically as a human organ, when its structure is altered, the development of non-communicable diseases characteristic of “dysbiosis” is favored (De Filippo et al., 2017; Baquero and Nombela, 2012; Gill et al., 2006). Gut microbiota direct normal intestinal development and physiology; it also impacts the function of “diffuse systems” within the host such as those responsible for immunity, metabolism, and epigenetic modification of the genome, and “distant” organs such as the brain (Flandroy et al., 2018; Chow et al., 2010; de Vos et al., 2022). Thus, changes in the composition or abundance of the microbiota may lead to various diseases such as Alzheimer’s, colorectal cancer, nonalcoholic fatty liver disease, inflammatory bowel disease, hypertension, bipolar disorder, and obesity (de Vos et al., 2022; Clemente et al., 2012; Chen et al., 2021; Góralczyk-Bińkowska et al., 2022).
Clearly, safeguarding the environment is inseparable from safeguarding human health. The objective of our contribution is to propose expanding the benefit-risk assessment process of weighing benefits (positive effects) and risks (potential harm) of the various medical options for the diagnosis, prevention, or treatment of a medical condition with the additional consideration of a given medical option’s potential negative impact on the environment and public health. We first provide an overview of the evidence on trace pharmaceutics in the environment and the regulatory and scientific context to help frame our proposal for an expanded benefit-risk assessment. Then, we describe the new perspective offered by the European Union Regulation 2017/745 on Medical Devices (EU MDR) for therapeutics made from natural substances. We follow this with an example pertaining to the treatment of patients with gastroesophageal reflux disease (GERD) and dyspepsia to help illustrate the expanded benefit-risk concept. Last, we discuss strategies for implementing scientific evidence from ecotoxicity studies into clinical practice so that it can be used by healthcare professionals and patients in their decision-making process.
2 Context for expanding the benefit-risk assessment
2.1 Identifying relevant literature
A comprehensive search of articles was conducted in the following databases from inception to January 2024: PubMed, Scopus, Science Direct, and Google Scholar. Our search strategy was based on the concepts of benefit-risk assessment, environmental impact of healthcare and pharmaceuticals, medical devices made of substances (MDMS), One Health and planetary health, and the interrelationship between human health-gut microbiome-environment. Keywords used in various combinations included benefit-risk assessment, sustainability, environment, environmental sustainability, environmental impact, ecotoxicity, biodegradability, bioaccumulation, contamination, One Health, planetary health, gut microbiome/microbiota, (complex) natural substances, medical devices made of substances/substance-based medical devices, pharmaceuticals, excipients, additives, preservatives, parabens, and artificial sweeteners. We also reviewed the reference lists of included articles for other potentially relevant sources.
2.2 Evidence on trace pharmaceutics in the environment
The issue of the environmental risk must be taken in account in the light of the emerging concerns for human health due to water pollution by pharmaceutical and their metabolites (World Health Organization, 2012; Stockholm County Council, 2014; Norman Network, 2023). While it is beyond the scope of our contribution to provide a detailed analysis of the level of environmental drug pollution, we believe it is necessary to share some topical elements of this issue for a better understanding of the need to expand the benefit-risk assessment.
2.2.1 Pharmaceutic residuals
Pharmaceutic residuals from the manufacturing process or related to patient use (i.e., patient urine and/or improper disposal of unused medication) enter the terrestrial and aquatic environments via wastewater (Moermond and de Rooy, 2022; Hofman-Caris et al., 2019; Molnarova et al., 2023). Unintended (additive) exposure in humans occurs through the drinking water supply and contamination of the food chain through root uptake of crops grown in soil irrigated with reclaimed wastewater or fertilized with biosolids from wastewater sludge (Miller et al., 2016; Yang et al., 2017; Dey et al., 2019; Paltiel et al., 2016). A review of studies conducted in Europe, Canada, the United States, and Brazil indicated that more than 80 pharmaceuticals, compounds, and drug metabolites have been detected up to the mg/L-level in sewage and surface waters (Heberer, 2002). Another review that analyzed over 200 studies of wastewater treatment plants (WWTPs) in Europe, Asia, and the United States found that the removal of pharmaceuticals varied between less than 15% to greater than 99% (Yang et al., 2017). Prescription categories of detected pharmaceuticals included analgesics, anti-inflammatory drugs, antibiotics, hormones, blood-lipid regulators, beta blockers, cytostatic drugs, and anti-epileptic drugs (Yang et al., 2017; Heberer, 2002). Variation in removal may be attributed to differences in removal efficiencies between treatment techniques implemented by WWTPs (Hofman-Caris et al., 2019; Dey et al., 2019).
The presence of pharmaceuticals and their residuals in the environment can have an adverse impact on nontarget organisms such as fish and amphibians, with a cascading effect on wider ecosystems (European Commission and Directorate General for Environment, 2020). Increasing amount of antibiotics into waters and soils creates a threat to all microorganisms in these environments, potentially accelerating the development, maintenance and spread of resistant bacteria and fungi (Cycoń et al., 2019; Kovalakova et al., 2020). Antibiotics can also be toxic to algae and cyanobacteria that form the base of the aquatic food chain, which in turn, can disrupt food webs (Kovalakova et al., 2020). Food webs can also be affected when a given pharmaceutical contaminant has different behavioral effects on prey and predators (Brodin et al., 2014).
2.2.2 The case of excipients
As with pharmaceuticals, there is also growing concern about the environmental accumulation of excipients. Common examples are artificial sweeteners and parabens, which are a group of substances commonly used as preservatives. Although excipients have been deemed safe for human consumption, knowledge of their overall safety is still evolving (Naik et al., 2021; Belton et al., 2020; Schiffman et al., 2023). With regards to artificial sweeteners, for example, degradation of acesulfame-potassium by UV light exposure can result in trace products that may cause oxidative stress in fish (Belton et al., 2020). Crustaceans (Daphnia magna) and gammarids (G. oceanicus, G. zaddachi) have been found to demonstrate behavioral changes after exposure to sucralose (e.g., feeding and finding shelter, spatial orientation, identifying and avoiding predators, swimming speed and height), which in turn may impact the food web (Wiklund et al., 2014; Wiklund et al., 2012). In the common carp, exposure to environmentally relevant concentrations of sucralose led to significant increases in lipid peroxidation, hydroperoxide content, and protein carbonyl content in the muscle, gill, and brain. Significant changes in antioxidant enzymatic activity in muscle and gills were also observed (Saucedo-Vence et al., 2017).
With respect to parabens, their impact on humans and other organisms is still under debate. These endocrine-disrupting compounds are widely detected in the environment (e.g., water resources, soil and sediments, air and dust, and biota) and in the human body (e.g., serum, breast tumor tissue, breastmilk, and placental tissue). There is some evidence from human studies to suggest that even low concentrations of paraben can influence organism homeostasis (Błędzka et al., 2014). Recently, the toxicity of three parabens (methylparaben, propylparaben, and butylparaben) was tested in two fish (Danio rerio and Cyprinus carpio) and one amphibian models (Xenopus laevis). The results indicated that gene expression was affected for detoxification, sex hormone production, or cell stress signaling.
Metabolites of excipients can also be detrimental. A recently published article reported findings from a series of eight in vitro tests on sucralose-6-acetate, a metabolite and impurity from the manufacturing process. The results indicated that sucralose-6-acetate is genotoxic; in vitro exposure of intestinal epithelium (with absence of intestinal bacteria) to sucralose-6-acetate damaged the integrity of the intestinal barrier function; sucralose-6-acetate induced the expression of intestinal epithelial genes that are associated with oxidative stress, inflammation, and cancer; sucralose-6-acetate blocked two members of a key family of enzymes that play a key role in detoxification (Schiffman et al., 2023).
2.2.3 Synergistic effects of contaminants
Another consideration is that synergistic effects of contaminants warrant proper attention. A recent study demonstrated that a synthetic hormone (17 alpha-ethinylestradiol, EE2) and a surfactant (sodium lauryl sulfate) not only had negative effects on mussel metabolism and oxidative status (Mytilus galloprovincialis) when acting alone but that the effect on behavior (i.e., valve closure) was greater in combination (Lopes et al., 2022). Furthermore, the findings from a proof-of-principle study involving a mixture of five steroid hormones on fish (i.e., fathead minnow) indicated that something can happen from “nothing”. That is, while each steroid hormone at low concentrations does not lead to a significant alteration in fish egg production on their own, simultaneous exposure of all five does have suppressive effects (Thrupp et al., 2018).
In sum, the long-term effect of additive exposure of trace quantities of pharmaceuticals and other chemicals to humans on development, disease resistance, and wellbeing is not known (Stockholm County Council, 2014). Nevertheless, it is clear risks exist and coordinated efforts to identify, measure, and mitigate these risks are needed. The entry of pharmaceutical pollutants into the water environment can be prevented with strategies to decrease input by producers, health professionals, and users and output loads by WWTPs (Moermond and de Rooy, 2022; Burns et al., 2018).
2.2.4 Main databases available for consultation
To enhance the availability of information throughout the healthcare chain regarding the behavior of different types of products and substances in the environment, there are various databases in existence and under development. Some databases (e.g., ECOdrug (Verbruggen et al., 2018), PubChem (National Institutes of Health and PubChem, 2024), ECOTOXicology Knowledgebase (Olker et al., 2022), CompTox Chemicals Dashboard (United States Environmental Protection Agency, 2023), NORMAN Database System (Norman, 2024), ECHA Chemical Database (European Chemicals Agency, 2024)) are particularly useful for guiding research in this field and to understand the environmental fate of substances used for human consumption. Another, namely, the Swedish Pharmaceuticals and Environment database (Stockholm County Council, 2014; Wennmalm and Gunnarsson, 2009; Janusinfo, 2023) represents a collaborative effort of different healthcare stakeholders to promote transparency about the environmental risk of pharmaceuticals. It was developed with the aim to stimulate health professionals and patients to use medicines with a lower environmental impact, and in turn, stimulate the pharmaceutical industry to design and develop more environmentally friendly products. All these examples can be accessed by the public. An overview of the aforementioned databases is provided in Table 1.
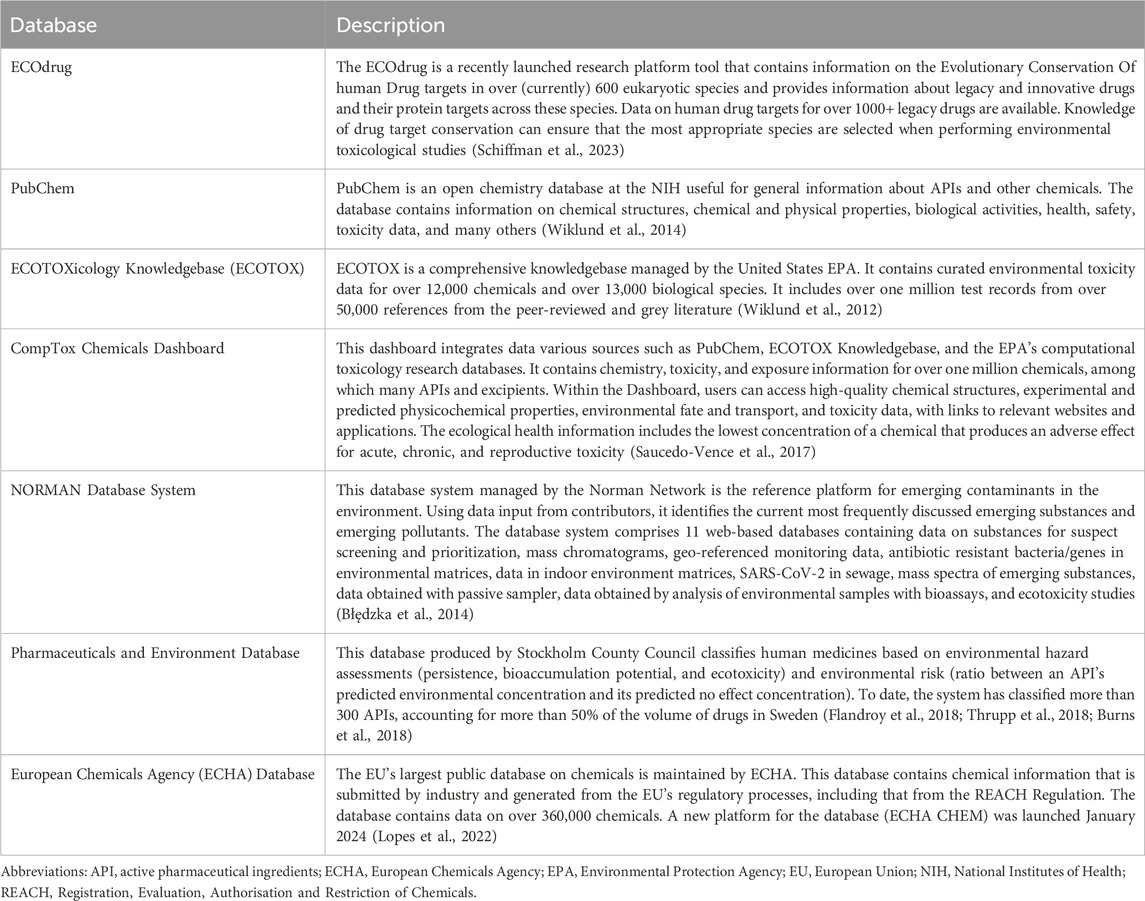
Table 1. Overview of open access databases containing ecotoxicity and environmental risk data of substances used in products for human consumption.
2.3 Regulatory and scientific context
Transdisciplinary movements advocating a paradigm shift from public health to planetary health are gaining traction and there is increasing awareness of the One Health approach and the interconnectedness between the environment, animals, and humans (Halonen et al., 2021; Mortimer and Pencheon, 2022; World Health Organization, 2022; Monath et al., 2010; Marselle et al., 2021). In addition, various policies at the international (e.g., EU Green Deal (European Commission, 2023a), UN SDGs (United Nations Department of Economic, 2023)) and national (e.g., National Health Service Net Zero in the UK (National Health Service, 2020), Green Deal 3.0 in the Netherlands (Government of the Netherlands, 2022)) level support efforts to measure and mitigate the unintended environmental impact of the healthcare sector.
In recent years, various publications have proposed ways to improve the regulatory guideline for environmental risk assessments (ERAs) of human medicinal products issued by the European Medicines Agency (EMA) (Gunnarsson et al., 2019; Gildemeister et al., 2023). The existing guideline (currently under review) lists all the tests that must be performed as part of the ERA (European Medicines Agency and Committee for Medicinal Products for Human Use CHMP, 2006; European Medicines Agency and Committee for Medicinal Products for Human Use CHMP, 2018).
Furthermore, in April 2023, the European Commission released a proposal for revising the general pharmaceutical legislation and promote innovation, particularly for unmet medical needs, while reducing regulatory burden and the environmental impact of medicines (European Commission, 2023b). The European Commission has recognized that the pharmaceutical product lifecycle can have negative impacts on the environment and is taking some steps toward a stricter regulation in the process of authorization, distribution, and maintenance in the market of the drugs.
2.4 Greener pharmacy
There is growing awareness among healthcare professionals that they are in a natural and favored position to inform the public and advocate for change to make healthcare more sustainable. For instance, the Pharmaceutical Group of the European Union (PGEU) has identified community pharmacists as having a key role in improving public health by providing information on the availability of “greener” pharmaceuticals, which have comparable evidence for efficacy and safety. This is in addition to advising on proper handling, adherence, and disposal (Pharmaceutial Group of European Union, 2019). Their contribution along with that of regulatory authorities and the scientific community of both editors and investigators, and consumers are critical. To practice sustainable evidence-based healthcare, health professionals and consumers also need information on a treatment’s environmental impact to supplement information on safety and performance.
3 Discussion
3.1 Expanding benefit-risk assessment of therapeutic products to include environmental effects
A benefit-risk assessment is a comparative evaluation or weighing of benefits (positive effects) and risks (potential harm) of various medical options for treatment, prophylaxis, prevention, or diagnosis and is essential for decision-making. Benefit-risk assessments have evolved from unstructured, subjective approaches to structured frameworks that can be descriptive or quantitative. A detailed overview of different methodologies is provided, for example, by Kürzinger et al. (2020).
Regardless of the specificities and strengths and limitations of the different methodologies, all aim to increase the transparency of decision-making process across the life cycle of a given medical option (Kürzinger et al., 2020). Benefits are usually defined as the successful treatment of the condition for which the drug is indicated as well as patient-related outcomes such as functional improvement or improved quality of life or patient satisfaction (Kürzinger et al., 2020; Kumar, 2020). Risks consist of adverse drug reactions, which can range from minor symptoms (e.g., headache, nausea, or dry mouth) to rare, severe reactions (e.g., liver failure, anaphylactic reaction, or cancer) (Kumar, 2020). To assist in informed decision-making, a benefit-risk assessment should always be conducted relative to no therapy, standard treatment, or a relevant comparator (Council for International Organizations of Medical Sciences CIOMS, 1998).
Various healthcare stakeholders (i.e., regulators, pharmaceutical companies, healthcare providers and their professional organizations, patients and support networks) are potential end-users of a benefit-risk assessment. Multiple public and private initiatives to developing benefit-risk frameworks and tools exist, including the development of a common benefit-risk assessment for all stakeholders (Walker et al., 2009). EMA and FDA have recently adopted the multi-criteria decision analysis framework that uses both qualitative and quantitative data (Chisholm et al., 2021). However, regardless of the framework or tools used, data on environmental health risks are not included on a standard basis.
Our proposed expanded benefit-risk assessment of therapeutic products has two pillars: 1) weighing the evidence of clinical safety and efficacy; and 2) evaluation of biodegradability of all compounds (actives and excipients) and their ecotoxicity to organisms that comprise an ecosystem with damage at different levels, e.g., molecular, cell, or tissue damage; development damage; damage with behavioral effects (Naik et al., 2021; Wiklund et al., 2014; Wiklund et al., 2012; Błędzka et al., 2014; Gunnarsson et al., 2019). Thus, in the situation where two or more treatments are comparable in terms of their clinical benefits and risks, then comparing the environmental data can help steer the choice to the more sustainable option. In the situation where the treatment option with a more favorable clinical benefit-risk profile has a greater environmental impact, mitigating strategies can then be considered and implemented.
It should be noted that although we have framed our proposal with the aim to affect the healthcare professionals and patients during their decision-making process, we also call on regulatory authorities and the scientific community to support this effort and contribute. When there is an environmental risk with a therapeutic product that has health benefits, then mitigating strategies need to be identified, developed, and implemented to minimize the impact on the planet. A broader benefit-risk assessment that includes environmental effects will help ensure we do not continue to turn a blind eye to the unintended effects of healthcare (Smith et al., 2022).
3.2 New perspectives: the use of natural substances in therapy
Therapeutic products based on natural substances, which have been developed within an allopathic and evidence-based practice approach, offer new perspectives for sustainable healthcare in line with the proposed expanded risk-benefit concept. The basis for this opportunity is provided, thanks in part to the certification of the EU MDR (European Parliament and Council of the European Union, 2017). In addition to reforming the medical device approval and post-marketing evaluation where clinical data are essential for demonstrating or confirming medical device conformity with relevant general safety and performance requirements, this regulation provides new provisions (i.e., specific classification rules and requirements) for MDMS. Of utmost importance, the EU MDR allows the certification of natural products whose mechanism of action is not linked to the specific interaction of an active molecule and its biological target (commonly a receptor), but rather on the interaction of the entire complex matrix.
One example involves the treatment of functional constipation in young children (age range: 6–48 months). Findings from a randomized controlled trial (RCT) comparing the CE-marked Promelaxin microenema made of 100% natural substances (Melilax, a class IIb MDMS) with the standard first-line treatment polyethylene glycol (PEG) 4,000 indicated that Promelaxin was non-inferior and that safety was comparable. Though only considered hypothesis-generating, the analysis of microbiota data suggested that Promelaxin may have a potentially lower impact on microbiota than PEG (Strisciuglio et al., 2021).
Below we provide a more extensive example of how a therapeutic product made of 100% natural substances was evaluated in terms of clinical safety, efficacy, and environmental risk using an evidence-based approach. We then use it to illustrate how the concept of an expanded risk-benefit approach can be applied to identify products that are safer for humans and the environment without depriving patients of necessary therapy.
3.2.1 Case example: poliprotect versus omeprazole
This example involves a mucosal protective agent (MPA) made of 100% natural substances (NeoBianacid, Aboca, Sansepolcro, Italy) and certified as class III medical device according to the EU MDR. This MPA is composed of Poliprotect (polysaccharide fraction from Aloe vera, Malva sylvestris, and Althea officinalis; minerals limestone and nahcolite) and a flavonoid fraction (from Glycyrrhiza glabra and Matricaria recutita). First, this MPA was compared to the standard of care (i.e., a proton-pump inhibitor, PPI; namely, omeprazole) in a double-blind, double-dummy, multicenter RCT (Corazziari et al., 2023). Then, the biodegradability of the two substances was investigated with an experiment conducted according to Organisation for Economic Co-operation and Development (OECD) test guidelines (OECD, 1992).
The efficacy and safety of the MPA were evaluated as compared with a standard dose of PPI (omeprazole 20 mg) in 273 endoscopy-negative patients with heartburn, a typical symptom of GERD and/or epigastric pain or burning (i.e., epigastric pain syndrome, EPS). In short, the primary efficacy endpoint was between-group comparison for the severity of heartburn and/or epigastric pain or burning from baseline to day 14 on a 100 mm visual analog scale. Secondary efficacy endpoints included comparison for change in symptom severity at earlier and later time points; use of rescue medicine; change from baseline in Quality of Life Index and Gastrointestinal Symptom Rating Scale score. Gut microbiota change was also assessed. Clinical safety was assessed by comparing number, proportion, and severity of total adverse events (AEs) as well as treatment related AEs, either overall or by System Organ Class, observed in each treatment group, and by means of routine blood and urine testing before and after treatment (Corazziari et al., 2023).
3.2.2 Proposed decision scheme
Based on preceding discussion, it is possible to hypothesize the following decision scheme to support the choice of therapeutic solutions in a prudent manner. The steps of the scheme are shown in Figure 1 and are summarized below.
Step 1: Comparing the efficacy and clinical safety of Poliprotect with omeprazole
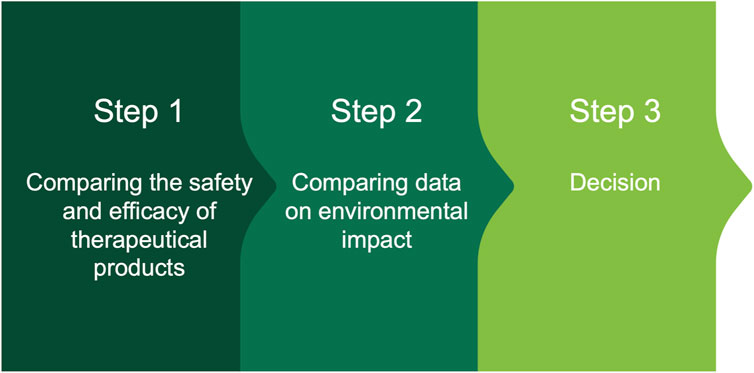
Figure 1. Decision scheme to support the choice of therapeutic solutions based on an assessment of safety, efficacy, and environmental impact.
In terms of clinical benefits, the MPA proved non-inferior to a standard dose of omeprazole for symptom relief, indicating MPA as a valid alternative treatment to PPI in managing EPS and heartburn in the absence of esophageal mucosal lesions. Also, the initial benefit obtained with daily MPA could be maintained with on-demand therapy. With regards to safety, no relevant AEs were reported in either group. Lastly, as for gut microbiota analysis, the use of PPI, as compared to MPA, was associated with a significantly higher over time dissimilarity due to oral cavity genera enrichment (Corazziari et al., 2023).
Step 2: Comparing data on environmental impact of Poliprotect versus omeprazole
A study using a suspect screening analysis to improve untargeted and targeted UHPLC-qToF approaches evaluated the biodegradability of Poliprotect and omeprazole. Results at day 28th indicated that Poliprotect is readily biodegradable and that omeprazole is not, aligning with previous independent environmental research data (IVL Swedish Environmental Research Institute, 1991). In the case of omeprazole, 11 transformation products were identified. Findings from untargeted analysis and suspects screening indicating incomplete mineralization of omeprazole were confirmed by targeted analysis (Mattoli et al., 2024). The toxicity on humans of omeprazole degradation products, to our knowledge, has resulted only from predictive in silico analyses, which were related to omeprazole forced degradation products (Shankar et al., 2019) and which may not correspond to those produced under normal conditions. Human urinary omeprazole metabolites may accumulate in surface water and wastewater, placing aquatic organisms at risk (Boix et al., 2014; Chiriac et al., 2024).
Step 3: Decision
Combining the evidence from steps one and two supports the choice for prescribing Poliprotect as opposed to omeprazole in patients with heartburn and functional painful dyspepsia. That is, Poliprotect is equally effective compared with omeprazole in terms of symptomatic relief and thanks to its biodegradability, its use will not contaminate the environment. Conversely, PPI remains the first-choice therapy of patients with erosive esophagitis and dyspeptic symptoms due to gastroduodenal lesions, provided the absence of evidence supporting a comparable safety and clinical efficacy for alternative therapy options.
3.3 Integrating persistence and ecotoxicity data into clinical practice
Addressing the environmental impact of healthcare is a complex issue, with multiple stakeholders, uncertainties, and no single, easy solution. As such, successful mitigation of healthcare’s unintended environmental effects requires all stakeholders in the value chain to be involved and contribute where they can contribute (Moermond and de Rooy, 2022), and that robust scientific data are available for evidence-based and data-driven decision-making. With our contribution, we would like to affect the healthcare professionals during their decision-making process with regards to which therapeutic options they will wish to prescribe or recommend, and with what sustainability implications. Namely, that when evidence for clinical performance and safety are comparable, they should also consider environmental safety. Ideally, they also involve their patients in this process. By doing so, together they can contribute to the solution by decreasing the input of pharmaceuticals into the wastewater system.
Additional strategies to facilitate the integration environmental issues of healthcare into daily clinical practices include the following:
– educating patients on limiting self-medicating purchases, reducing the storage of excessive stocks of medicines at home, and proper disposal practices of unused and unwanted pharmaceuticals;
– including environmental aspects of pharmaceuticals in professional practice guidelines and information materials for healthcare professionals and patients. This may be facilitated by including environmental/ecotoxicity specialists in the team that is developing and updating such documents;
– embedding One Health, planetary health, and environmental aspects of medical products in the training of all healthcare professionals and continuing education programs (Pharmaceutial Group of European Union, 2019);
– leveraging informatics, for example, by integrating environmental data into online tools for clinicians such as electronic medication management systems (Smith et al., 2022). For example, in cases where there are two or more therapeutic options with comparable efficacy and safety, the default recommendation or prescription may be set to the one with a smaller environmental impact. When comparative data of different therapeutic options are not available, then the program could be set to alert healthcare providers to discuss mitigating strategies such as urine collection (such in the case of oncologic drugs that must be inactivated before disposal in the sewer system) or proper disposal with their patients.
4 Conclusion
While ensuring that every patient gets the treatment they deserve, it is essential to safeguard the health of the entire human population by protecting the environment and biodiversity. The case example of MPA illustrates that products with comparable performance and human safety can have different environmental impacts. As prescribers and patient educators, healthcare professionals can have a critical role in increasing the market demand for environmentally friendly products and preventing the entry of pharmaceutical residuals into water systems. In conclusion, we propose that expanding the benefit-risk assessment to include data on environmental impact during clinical decision-making is a way to achieve a healthier outcome for all.
Author contributions
EG: Conceptualization, Supervision, Writing–original draft, Writing–review and editing. LM: Conceptualization, Investigation, Visualization, Writing–original draft, Writing–review and editing. AC: Conceptualization, Investigation, Project administration, Writing–original draft, Writing–review and editing. VM: Conceptualization, Investigation, Writing–original draft, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Medical writing support was provided by LS Academy; funding for this assistance was provided by Aboca S.p.A.
Conflict of interest
Authors EG, LM, and AC are employed by Aboca S.p.A., Loc. Aboca. VM provides consulting services to Aboca S.p.A. Società Agricola.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
API, active pharmaceutical ingredient; EMA, European Medicines Agency; EPA, Environmental Protection Agency; EPS, epigastric pain syndrome; EU MDR, European Union Regulation 2017/745 on Medical Devices; GERD, gastroesophageal reflux disease; MDMS, medical device made of substances; MPA, mucosal protective agent; OECD, Organisation for Economic Co-operation and Development; PEG, polyethylene glycol; PGEU, Pharmaceutial Group of European Union; PPI, proton-pump inhibitor; RCT, randomized controlled trial.
References
Belton, K., Schaefer, E., and Guiney, P. D. (2020). A review of the environmental fate and effects of acesulfame-potassium. Integr. Environ. Assess. Manag. 16 (4), 421–437. doi:10.1002/ieam.4248
PubMed Abstract | CrossRef Full Text | Google Scholar
Boix, C., Ibáñez, M., Zamora, T., Sancho, J. V., Niessen, W. M., and Hernández, F. (2014). Identification of new omeprazole metabolites in wastewaters and surface waters. Sci. Total Environ. 468-469, 706–714. doi:10.1016/j.scitotenv.2013.08.095
PubMed Abstract | CrossRef Full Text | Google Scholar
Brodin, T., Piovano, S., Fick, J., Klaminder, J., Heynen, M., and Jonsson, M. (2014). Ecological effects of pharmaceuticals in aquatic systems–impacts through behavioural alterations. Philos. Trans. R. Soc. Lond B Biol. Sci. 369 (1656), 20130580. doi:10.1098/rstb.2013.0580
PubMed Abstract | CrossRef Full Text | Google Scholar
Burns, E. E., Carter, L. J., Snape, J., Thomas-Oates, J., and Boxall, A. B. A. (2018). Application of prioritization approaches to optimize environmental monitoring and testing of pharmaceuticals. J. Toxicol. Environ. Health B Crit. Rev. 21 (3), 115–141. doi:10.1080/10937404.2018.1465873
PubMed Abstract | CrossRef Full Text | Google Scholar
Chiriac, F. L., Paun, I., Iancu, V. I., Pirvu, F., Dinu, C., Niculescu, M., et al. (2024). Fate of pharmaceutical residue in two Romanian rivers receiving treated water: occurrence, distribution and risk assessment. Sci. Total Environ. 923, 171359. doi:10.1016/j.scitotenv.2024.171359
PubMed Abstract | CrossRef Full Text | Google Scholar
Chisholm, O., Sharry, P., and Phillips, L. (2021). Multi-criteria decision analysis for benefit-risk analysis by national regulatory authorities. Front. Med. (Lausanne) 8, 820335. doi:10.3389/fmed.2021.820335
PubMed Abstract | CrossRef Full Text | Google Scholar
Chow, J., Lee, S. M., Shen, Y., Khosravi, A., and Mazmanian, S. K. (2010). Host-bacterial symbiosis in health and disease. Adv. Immunol. 107, 243–274. doi:10.1016/b978-0-12-381300-8.00008-3
PubMed Abstract | CrossRef Full Text | Google Scholar
Clemente, J. C., Ursell, L. K., Parfrey, L. W., and Knight, R. (2012). The impact of the gut microbiota on human health: an integrative view. Cell 148 (6), 1258–1270. doi:10.1016/j.cell.2012.01.035
PubMed Abstract | CrossRef Full Text | Google Scholar
Corazziari, E. S., Gasbarrini, A., D’Alba, L., D’Ovidio, V., Riggio, O., Passaretti, S., et al. (2023). Poliprotect vs omeprazole in the relief of heartburn, epigastric pain, and burning in patients without erosive esophagitis and gastroduodenal lesions: a randomized, controlled trial. Am. J. Gastroenterol. 118, 2014–2024. doi:10.14309/ajg.0000000000002360
PubMed Abstract | CrossRef Full Text | Google Scholar
Cycoń, M., Mrozik, A., and Piotrowska-Seget, Z. (2019). Antibiotics in the soil environment-degradation and their impact on microbial activity and diversity. Front. Microbiol. 10, 338. doi:10.3389/fmicb.2019.00338
PubMed Abstract | CrossRef Full Text | Google Scholar
De Filippo, C., Di Paola, M., Ramazzotti, M., Albanese, D., Pieraccini, G., Banci, E., et al. (2017). Diet, environments, and gut microbiota. A preliminary investigation in children living in rural and urban Burkina Faso and Italy. Front. Microbiol. 8, 1979. doi:10.3389/fmicb.2017.01979
PubMed Abstract | CrossRef Full Text | Google Scholar
Dey, S., Bano, F., and Malik, A. (2019). “Pharmaceuticals and personal care product (PPCP) contamination—a global discharge inventory,” in Pharmaceutical and personal care products waste management and treatment technology: emerging contaminants and micro pollutants. Editors M. N. V. Prasad, M. Vithanage, and A. Kapley (Elsevier), 1–26. doi:10.1016/B978-0-12-816189-0.00001-9
CrossRef Full Text | Google Scholar
European Commission, Directorate General for Environment (2020). Update on progress and implementation: European union strategic approach to pharmaceuticals in the environment. Luxembourg: European Union. doi:10.2779/037747
CrossRef Full Text | Google Scholar
European Medicines Agency, Committee for Medicinal Products for Human Use (CHMP). Guideline on the environmental risk assessment of medical products for human use. 2006;
Google Scholar
European Medicines Agency, Committee for Medicinal Products for Human Use (CHMP) (2018) Guideline on the environmental risk assessment of medicinal products for human use – draft, 1. Amsterdam, Netherlands: EMEA/CHMP/SWP/4447/00 Rev.
Google Scholar
European Parliament, Council of the European Union (2017). Regulation (EU) 2017/745 of the European parliament and of the Council of 5 April 2017 on medical devices, amending directive 2001/83/EC, regulation (EC) No 178/2002 and regulation (EC) No 1223/2009 and repealing Council directives 90/385/EEC and 93/42/EEC (pdf). Available at:
Google Scholar
Flandroy, L., Poutahidis, T., Berg, G., Clarke, G., Dao, M. C., Decaestecker, E., et al. (2018). The impact of human activities and lifestyles on the interlinked microbiota and health of humans and of ecosystems. Sci. Total Environ. 627, 1018–1038. doi:10.1016/j.scitotenv.2018.01.288
PubMed Abstract | CrossRef Full Text | Google Scholar
Gildemeister, D., Moermond, C. T. A., Berg, C., Bergstrom, U., Bielská, L., Evandri, M. G., et al. (2023). Improving the regulatory environmental risk assessment of human pharmaceuticals: required changes in the new legislation. Regul. Toxicol. Pharmacol. 142, 105437. doi:10.1016/j.yrtph.2023.105437
PubMed Abstract | CrossRef Full Text | Google Scholar
Gill, S. R., Pop, M., Deboy, R. T., Eckburg, P. B., Turnbaugh, P. J., Samuel, B. S., et al. (2006). Metagenomic analysis of the human distal gut microbiome. Science 312 (5778), 1355–1359. doi:10.1126/science.1124234
PubMed Abstract | CrossRef Full Text | Google Scholar
Góralczyk-Bińkowska, A., Szmajda-Krygier, D., and Kozłowska, E. (2022). The microbiota-gut-brain axis in psychiatric disorders. Int. J. Mol. Sci. 23 (19), 11245. doi:10.3390/ijms231911245
PubMed Abstract | CrossRef Full Text | Google Scholar
Grenni, P., Ancona, V., and Caracciolo, A. B. (2018). Ecological effects of antibiotics on natural ecosystems: a review. Microchem. J. 236, 25–39. doi:10.1016/j.microc.2017.02.006
CrossRef Full Text | Google Scholar
Gunnarsson, L., Snape, J. R., Verbruggen, B., Owen, S. F., Kristiansson, E., Margiotta-Casaluci, L., et al. (2019). Pharmacology beyond the patient – the environmental risks of human drugs. Environ. Int. 129, 320–332. doi:10.1016/j.envint.2019.04.075
PubMed Abstract | CrossRef Full Text | Google Scholar
Halonen, J. I., Erhola, M., Furman, E., Haahtela, T., Jousilahti, P., Barouki, R., et al. (2021). A call for urgent action to safeguard our planet and our health in line with the Helsinki Declaration. Environ. Res. 193, 110600. doi:10.1016/j.envres.2020.110600
PubMed Abstract | CrossRef Full Text | Google Scholar
Heberer, T. (2002). Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: a review of recent research data. Toxicol. Lett. 131 (1-2), 5–17. doi:10.1016/s0378-4274(02)00041-3
PubMed Abstract | CrossRef Full Text | Google Scholar
Hirt, H. (2020). Healthy soils for healthy plants for healthy humans: how beneficial microbes in the soil, food and gut are interconnected and how agriculture can contribute to human health. EMBO Rep. 21 (8), e51069. doi:10.15252/embr.202051069
PubMed Abstract | CrossRef Full Text | Google Scholar
Hofman-Caris, R., ter Laak, T., Huiting, H., Tolkamp, H., de Man, A., van Diepenbeek, P., et al. (2019). Origin, fate and control of pharmaceuticals in the urban water cycle: a case study. Water 11, 1034. doi:10.3390/w11051034
CrossRef Full Text | Google Scholar
IVL Swedish Environmental Research Institute. Investigation of the “ready biodegradability” of A 001 drug substance, 1991.
Google Scholar
Kovalakova, P., Cizmas, L., McDonald, T. J., Marsalek, B., Feng, M., and Sharma, V. K. (2020). Occurrence and toxicity of antibiotics in the aquatic environment: a review. Chemosphere 251, 126351. doi:10.1016/j.chemosphere.2020.126351
PubMed Abstract | CrossRef Full Text | Google Scholar
Kürzinger, M. L., Douarin, L., Uzun, I., El-Haddad, C., Hurst, W., Juhaeri, J., et al. (2020). Structured benefit-risk evaluation for medicinal products: review of quantitative benefit-risk assessment findings in the literature. Ther. Adv. Drug Saf. 11, 2042098620976951. doi:10.1177/2042098620976951
PubMed Abstract | CrossRef Full Text | Google Scholar
Lenzen, M., Malik, A., Li, M., Fry, J., Weisz, H., Pichler, P. P., et al. (2020). The environmental footprint of health care: a global assessment. Lancet Planet Health 4 (7), e271–e279. doi:10.1016/s2542-5196(20)30121-2
PubMed Abstract | CrossRef Full Text | Google Scholar
Li, S., Liu, Y., Wu, Y., Hu, J., Zhang, Y., Sun, Q., et al. (2022). Antibiotics in global rivers. Natl. Sci. Open 1 (2), 20220029. doi:10.1360/nso/20220029
CrossRef Full Text | Google Scholar
Lopes, J., Coppola, F., Russo, T., Maselli, V., Di Cosmo, A., Meucci, V., et al. (2022). Behavioral, physiological and biochemical responses and differential gene expression in Mytilus galloprovincialis exposed to 17 alpha-ethinylestradiol and sodium lauryl sulfate. J. Hazard Mater. 426, 128058. doi:10.1016/j.jhazmat.2021.128058
PubMed Abstract | CrossRef Full Text | Google Scholar
Maghsodian, Z., Sanati, A. M., Mashifana, T., Sillanpää, M., Feng, S., Nhat, T., et al. (2022). Occurrence and distribution of antibiotics in the water, sediment, and biota of freshwater and marine environments:a review. Antibiot. (Basel) 11 (11), 1461. doi:10.3390/antibiotics11111461
PubMed Abstract | CrossRef Full Text | Google Scholar
Marselle, M. R., Hartig, T., Cox, D. T. C., de Bell, S., Knapp, S., Lindley, S., et al. (2021). Pathways linking biodiversity to human health: a conceptual framework. Environ. Int. 150, 106420. doi:10.1016/j.envint.2021.106420
PubMed Abstract | CrossRef Full Text | Google Scholar
Mattoli, L., Proietti, G., Fodaroni, G., Quintiero, C. M., Burico, M., Gianni, M., et al. (2024). Suspect screening analysis to improve untargeted and targeted UHPLC-qToF approaches: the biodegradability of a proton pump inhibitor medicine and a natural medical device. Sci. Rep. 14 (1), 51. doi:10.1038/s41598-023-49948-8
PubMed Abstract | CrossRef Full Text | Google Scholar
Miller, E. L., Nason, S. L., Karthikeyan, K. G., and Pedersen, J. A. (2016). Root uptake of pharmaceuticals and personal care product ingredients. Environ. Sci. Technol. 50 (2), 525–541. doi:10.1021/acs.est.5b01546
PubMed Abstract | CrossRef Full Text | Google Scholar
Moermond, C. T. A., and de Rooy, M. (2022). The Dutch chain approach on pharmaceuticals in water: stakeholders acting together to reduce the environmental impact of pharmaceuticals. Br. J. Clin. Pharmacol. 88 (12), 5074–5082. doi:10.1111/bcp.15509
PubMed Abstract | CrossRef Full Text | Google Scholar
Molnarova, L., Halesova, T., Vaclavikova, M., and Bosakova, Z. (2023). Monitoring pharmaceuticals and personal care products in drinking water samples by the LC-MS/MS method to estimate their potential health risk. Molecules 28 (15), 5899. doi:10.3390/molecules28155899
PubMed Abstract | CrossRef Full Text | Google Scholar
Naik, A. Q., Zafar, T., and Shrivastava, V. K. (2021). Environmental impact of the presence, distribution, and use of artificial sweeteners as emerging sources of pollution. J. Environ. Public Health 2021, 6624569. doi:10.1155/2021/6624569
PubMed Abstract | CrossRef Full Text | Google Scholar
OECD (1992). “Test No. 301: ready biodegradability,” in OECD guidelines for the testing of chemicals, section 3. Paris: OECD Publishing. doi:10.1787/9789264070349-en
CrossRef Full Text | Google Scholar
Olker, J. H., Elonen, C. M., Pilli, A., Anderson, A., Kinziger, B., Erickson, S., et al. (2022). The ECOTOXicology knowledgebase: a curated database of ecologically relevant toxicity tests to support environmental research and risk assessment. Environ. Toxicol. Chem. 41 (6), 1520–1539. doi:10.1002/etc.5324
PubMed Abstract | CrossRef Full Text | Google Scholar
Paltiel, O., Fedorova, G., Tadmor, G., Kleinstern, G., Maor, Y., and Chefetz, B. (2016). Human exposure to wastewater-derived pharmaceuticals in fresh produce: a randomized controlled trial focusing on carbamazepine. Environ. Sci. Technol. 50 (8), 4476–4482. doi:10.1021/acs.est.5b06256
PubMed Abstract | CrossRef Full Text | Google Scholar
Prescott, S. L., Logan, A. C., Bristow, J., Rozzi, R., Moodie, R., Redvers, N., et al. (2022). Exiting the Anthropocene: achieving personal and planetary health in the 21st century. Allergy 77 (12), 3498–3512. doi:10.1111/all.15419
PubMed Abstract | CrossRef Full Text | Google Scholar
Robles-Jimenez, L. E., Aranda-Aguirre, E., Castelan-Ortega, O. A., Shettino-Bermudez, B. S., Ortiz-Salinas, R., Miranda, M., et al. (2021). Worldwide traceability of antibiotic residues from livestock in wastewater and soil:a systematic review. Anim. (Basel) 12 (1), 60. doi:10.3390/ani12010060
PubMed Abstract | CrossRef Full Text | Google Scholar
Romanelli, C., Cooper, D., Campbell-Lendrum, D., Maiero, M., Karesh, W. B., Hunter, D., et al. (2015). Connecting global priorities: biodiversity and human health: a state of knowledge review. Geneva, Switzerland: World Health Organization.
Google Scholar
Saucedo-Vence, K., Elizalde-Velázquez, A., Dublán-García, O., Galar-Martínez, M., Islas-Flores, H., SanJuan-Reyes, N., et al. (2017). Toxicological hazard induced by sucralose to environmentally relevant concentrations in common carp (Cyprinus carpio). Sci. Total Environ. 575, 347–357. doi:10.1016/j.scitotenv.2016.09.230
PubMed Abstract | CrossRef Full Text | Google Scholar
Schiffman, S. S., Scholl, E. H., Furey, T. S., and Nagle, H. T. (2023). Toxicological and pharmacokinetic properties of sucralose-6-acetate and its parent sucralose: in vitro screening assays. J. Toxicol. Environ. Health B Crit. Rev. 26 (6), 307–341. doi:10.1080/10937404.2023.2213903
PubMed Abstract | CrossRef Full Text | Google Scholar
Shankar, G., Borkar, R. M., Udutha, S., Kanakaraju, M., Sai Charan, G., Misra, S., et al. (2019). Identification and structural characterization of the stress degradation products of omeprazole using Q-TOF-LC-ESI-MS/MS and NMR experiments: evaluation of the toxicity of the degradation products. New J. Chem. 43 (19), 7185–7189. doi:10.1039/C8NJ05561K
CrossRef Full Text | Google Scholar
Smith, C. L., Zurynski, Y., and Braithwaite, J. (2022). We can’t mitigate what we don’t monitor: using informatics to measure and improve healthcare systems’ climate impact and environmental footprint. J. Am. Med. Inf. Assoc. 29 (12), 2168–2173. doi:10.1093/jamia/ocac113
PubMed Abstract | CrossRef Full Text | Google Scholar
Strisciuglio, C., Coppola, V., Russo, M., Tolone, C., Marseglia, G. L., Verrotti, A., et al. (2021). Promelaxin microenemas are non-inferior to oral polyethylene glycol for the treatment of functional constipation in young children: a randomized clinical trial. Front. Pediatr. 9, 753938. doi:10.3389/fped.2021.753938
PubMed Abstract | CrossRef Full Text | Google Scholar
Symochko, L., Demyanyuk, O., Symochko, V., Grulova, D., Fejer, J., and Mariychuk, R. (2023). The spreading of antibiotic-resistant bacteria in terrestrial ecosystems and the formation of soil resistome. Land 12 (4), 769. doi:10.3390/land12040769
CrossRef Full Text | Google Scholar
Thrupp, T. J., Runnalls, T. J., Scholze, M., Kugathas, S., Kortenkamp, A., and Sumpter, J. P. (2018). The consequences of exposure to mixtures of chemicals: something from ‘nothing’ and ‘a lot from a little’ when fish are exposed to steroid hormones. Sci. Total Environ. 619-620, 1482–1492. doi:10.1016/j.scitotenv.2017.11.081
PubMed Abstract | CrossRef Full Text | Google Scholar
United Nations department of economic and social affairs, the 17 goals. Available at: (Accessed 9 October 2023).
Verbruggen, B., Gunnarsson, L., Kristiansson, E., Österlund, T., Owen, S. F., Snape, J. R., et al. (2018). ECOdrug: a database connecting drugs and conservation of their targets across species. Nucleic Acids Res. 46 (D1), D930-D936–d6. doi:10.1093/nar/gkx1024
PubMed Abstract | CrossRef Full Text | Google Scholar
Walker, S., McAuslane, N., Liberti, L., and Salek, S. (2009). Measuring benefit and balancing risk: strategies for the benefit-risk assessment of new medicines in a risk-averse environment. Clin. Pharmacol. Ther. 85 (3), 241–246. doi:10.1038/clpt.2008.277
PubMed Abstract | CrossRef Full Text | Google Scholar
Wennmalm, A., and Gunnarsson, B. (2009). Pharmaceutical management through environmental product labeling in Sweden. Environ. Int. 35 (5), 775–777. doi:10.1016/j.envint.2008.12.008
PubMed Abstract | CrossRef Full Text | Google Scholar
Wiklund, A. E., Adolfsson-Erici, M., Liewenborg, B., and Gorokhova, E. (2014). Sucralose induces biochemical responses in Daphnia magna. PLoS One 9 (4), e92771. doi:10.1371/journal.pone.0092771
PubMed Abstract | CrossRef Full Text | Google Scholar
Wiklund, A. K., Breitholtz, M., Bengtsson, B. E., and Adolfsson-Erici, M. (2012). Sucralose – an ecotoxicological challenger? Chemosphere 86 (1), 50–55. doi:10.1016/j.chemosphere.2011.08.049
PubMed Abstract | CrossRef Full Text | Google Scholar
Yang, Y., Ok, Y. S., Kim, K. H., Kwon, E. E., and Tsang, Y. F. (2017). Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: a review. Sci. Total Environ. 596-597, 303–320. doi:10.1016/j.scitotenv.2017.04.102
PubMed Abstract | CrossRef Full Text | Google Scholar
link